
How do I manage translation projects?
The translation of medical, technical and scientific documents is a complex process. Like a surgical operation or the development of a new drug, it entails many steps and precautions to ensure accurate and safe results.
Have a look at my translation procedure - perfected during 20 years of experience as a translator.
Have a look at my translation procedure - perfected during 20 years of experience as a translator.
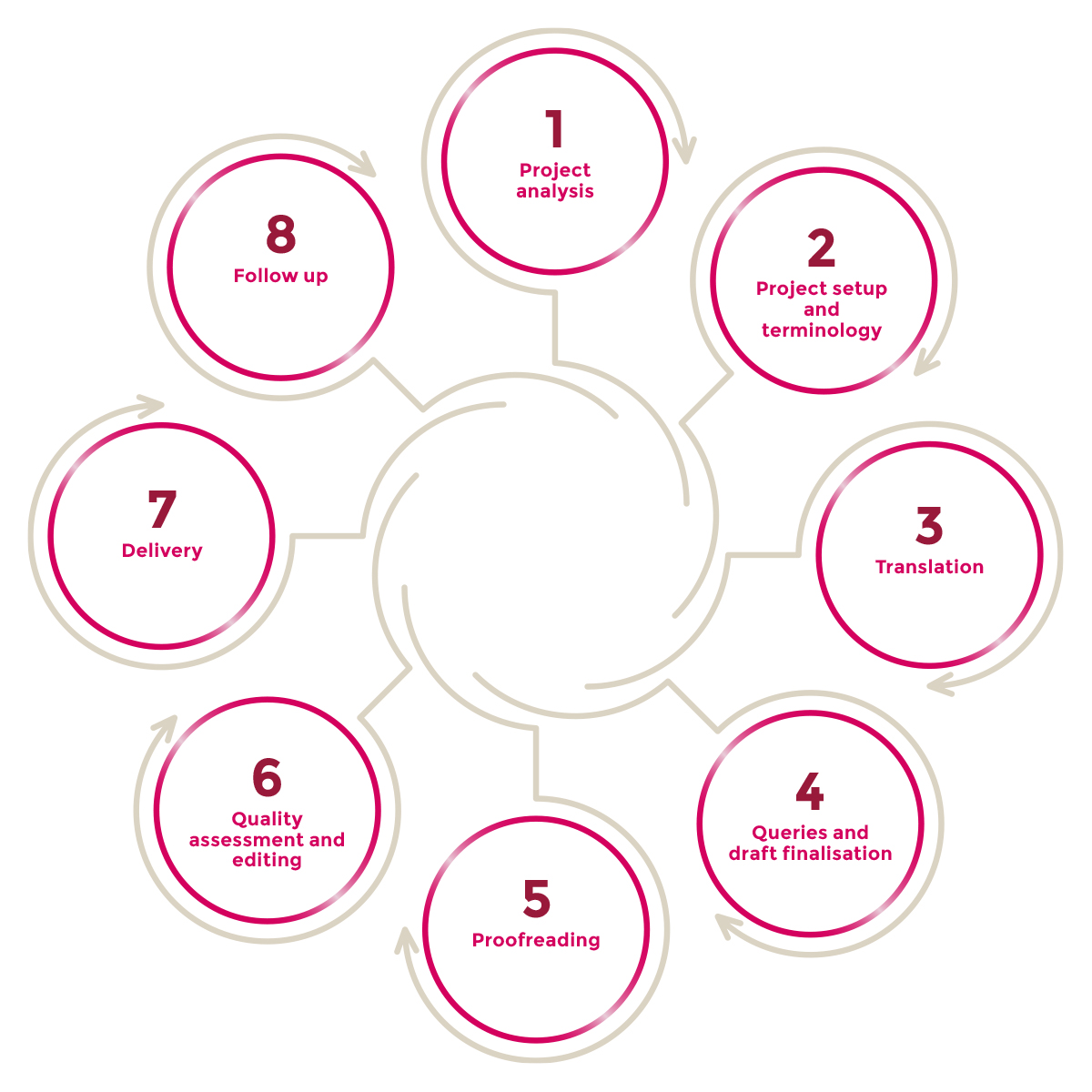
- Project Analysis
- Project setup and terminology
- Translation
- Queries and draft finalisation
- Proofreading
- Quality assessment and editing
- Delivery
- Follow up
• Project assessment: scope, target audience, formats, specifications.
• Source text analysis.
• Analysis of reference materials provided by the client.
• Source text analysis.
• Analysis of reference materials provided by the client.
• Technical project setup: text extraction, file conversion and document setting.
Project-specific information sourcing (e.g. glossaries, regulatory specifications, templates, technical standards).
• Analysis of client-specific glossary (when available) or setting up a customised glossary.
• Terminology-related research.
Project-specific information sourcing (e.g. glossaries, regulatory specifications, templates, technical standards).
• Analysis of client-specific glossary (when available) or setting up a customised glossary.
• Terminology-related research.
• Translation of English source text into Italian, focussing on accuracy and consistency in terms of style, terminology and format.
• Where needed, compliance assessment of the translation against current standards (e.g. Regulation (EU) no. 536/2014 for the translation of clinical trial documentation; EMA templates for package inserts and SPCs; etc.).
• Where needed, compliance assessment of the translation against current standards (e.g. Regulation (EU) no. 536/2014 for the translation of clinical trial documentation; EMA templates for package inserts and SPCs; etc.).
• When required, queries are sent to clients or industry experts. Over time, I have developed a network of trusted colleagues and medical experts that can help me clarify ambiguities and fill knowledge gaps. With the utmost attention to the confidentiality of data.
• Creating the final draft, based on clients’ and experts’ feedback.
• Creating the final draft, based on clients’ and experts’ feedback.
• Review of the Italian text (with parallel English source) by me. The translation is assessed for completeness, clarity, readability, style and tone.
• Review by a peer (when agreed upon).
• I may also ask a peer to review the text (subject to the client’s approval) when an extra pair of eyes is required.
• Review by a peer (when agreed upon).
• I may also ask a peer to review the text (subject to the client’s approval) when an extra pair of eyes is required.
• QA check, carried out using professional software. Typical issues that are checked: grammar and syntax; numbers and mathematical symbols; terminology, style and tone; measure units and conversions; punctuation; tabs and spacing.
• In-layout proofreading (e.g. for PowerPoint files).
• Final reading of the translation (target text only, printed). To protect the confidentiality of the data, printed copies are shredded upon delivery of the project.
• In-layout proofreading (e.g. for PowerPoint files).
• Final reading of the translation (target text only, printed). To protect the confidentiality of the data, printed copies are shredded upon delivery of the project.
• Delivery of the translation no later than the agreed delivery date.
• The documents are delivered as per project specifications.
• When required, the translations are supplemented with notes, comments and highlighting.
• The documents are delivered as per project specifications.
• When required, the translations are supplemented with notes, comments and highlighting.
• Receiving and analysing client feedback.
• Updating client-specific glossaries.
• Updating client-specific glossaries.
